[Policy Recommendation] Ensuring Equitable Access to Cancer Gene Panel Testing and Key Considerations for Applying the Mixed Medical Services Program (April 28, 2025)
date : 7/14/2025
Tags: Cancer, NCDs, Precision Cancer Medicine
![[Policy Recommendation] Ensuring Equitable Access to Cancer Gene Panel Testing and Key Considerations for Applying the Mixed Medical Services Program (April 28, 2025)](https://hgpi.org/en/wp-content/uploads/sites/2/ncd-pcm-20250430-top-1.jpg)
The English version of the report has been published. (July 14, 2025)
Health and Global Policy Institute (HGPI) has released a new policy recommendation titled “Ensuring Equitable Access to Cancer Gene Panel Testing and Key Considerations for Applying the Mixed Medical Services Program” on April 28, 2025.
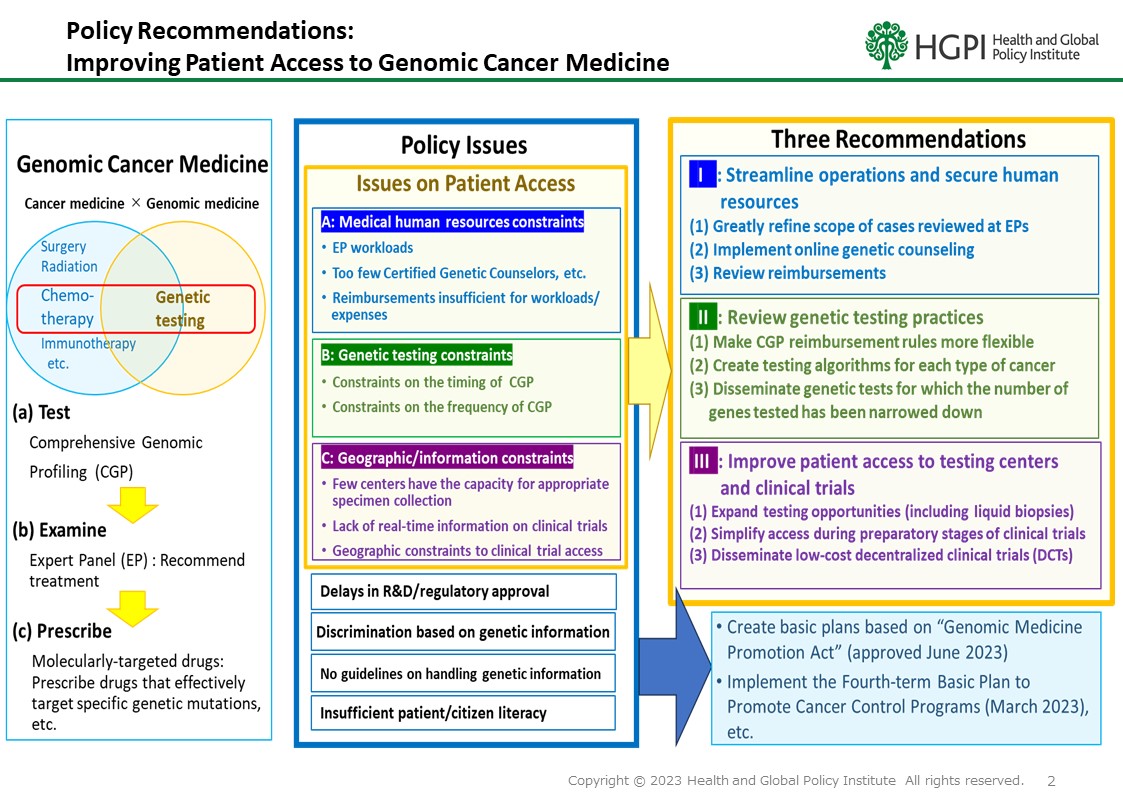
This recommendation addresses institutional issues in Japan’s cancer genomic medicine system, where cancer gene panel testing (CGP) is, in principle, limited to patients who have completed standard treatments. As a result, optimal treatment opportunities are sometimes missed, creating disparities in access among patients. Furthermore, ongoing discussions regarding the application of Japan’s Mixed Medical Services Program—a regulatory framework allowing partial integration of insured and uninsured treatments—raise additional concerns about equity and sustainability.
HGPI views genomic medicine not as a high-level, niche technology but as an essential component of cancer care that should benefit all people. Building on past research, multistakeholder dialogues, and evidence from domestic and international sources, this recommendation aims to accelerate policy reform that places patient-centeredness and access equity at its core.
Overview of the Recommendation
Perspective 1
Perspective 2
Abolish the current restriction that limits CGP to patients who have completed (or are expected to complete) standard treatment under the national reimbursement system. Testing should be made available at an appropriate time in accordance with relevant clinical guidelines, enabling patients to receive the right treatment at the right time.
When considering the application of CGP from the initial treatment phase under the Mixed Medical Services Program, the discussion must recognize that CGP is already being used to select approved standard therapies and that significant socioeconomic disparities exist in private insurance coverage. Public coverage should remain the foundational goal.Perspective 3
As a transitional measure, Japan should promote the development of cross-drug companion diagnostics. Simultaneously, policymakers should continue to address the systemic divide between CGP and companion diagnostics under the reimbursement structure and prepare for future integration with whole genome sequencing.
This recommendation serves as a policy roadmap for ensuring that all patients—regardless of income level or treatment institution—have fair and timely access to cancer genomic testing and the therapeutic opportunities it enables.
Top Research & Recommendations Posts
- [Policy Recommendations] The Path to a Sustainable Healthcare System: Three Key Objectives for Public Deliberation (January 22, 2026)
- [Research Report] The 2025 Public Opinion Survey on Healthcare in Japan (March 17, 2025)
- [Research Report] Perceptions, Knowledge, Actions and Perspectives of Healthcare Organizations in Japan in Relation to Climate Change and Health: A Cross-Sectional Study (November 13, 2025)
- [Policy Recommendations] Reshaping Japan’s Immunization Policy for Life Course Coverage and Vaccine Equity: Challenges and Prospects for an Era of Prevention and Health Promotion (April 25, 2025)
- [Research Report] The 2023 Public Opinion Survey on Satisfaction in Healthcare in Japan and Healthcare Applications of Generative AI (January 11, 2024)
- [Research Report] AMR Policy Update #4: Cancer Care and AMR (Part 1)
- [Public Comment Submission] “Assessment Report on Climate Change Impacts in Japan (Draft Overview)” (December 24, 2025)
- [Policy Recommendations] Developing a National Health and Climate Strategy for Japan (June 26, 2024)
- [Research Report] The Public Opinion Survey on Child-Rearing in Modern Japan (Final Report) (March 4, 2022)
- [Research Report] Survey of Japanese Physicians Regarding Climate Change and Health (December 3, 2023)
Featured Posts
-
2026-01-09
[Registration Open] (Hybrid Format) Dementia Project FY2025 Initiative Concluding Symposium “The Future of Dementia Policy Surrounding Families and Others Who Care for People with Dementia” (March 9, 2026)
![[Registration Open] (Hybrid Format) Dementia Project FY2025 Initiative Concluding Symposium “The Future of Dementia Policy Surrounding Families and Others Who Care for People with Dementia” (March 9, 2026)](https://hgpi.org/en/wp-content/uploads/sites/2/dementia-20260309-top.png)
-
2026-02-05
[Registration Open] (Webinar) The 141st HGPI Seminar “Current Status and Future Prospects of Korea’s Obesity Policy: Voices of People with Lived Experience in Policy Promotion” (March 3, 2026)
![[Registration Open] (Webinar) The 141st HGPI Seminar “Current Status and Future Prospects of Korea’s Obesity Policy: Voices of People with Lived Experience in Policy Promotion” (March 3, 2026)](https://hgpi.org/en/wp-content/uploads/sites/2/hs141-top-1.png)
-
2026-02-06
[Research Report] AMR Policy Update #5: Cancer Care and AMR (Part 2)
![[Research Report] AMR Policy Update #5: Cancer Care and AMR (Part 2)](https://hgpi.org/en/wp-content/uploads/sites/2/HGPI_20260204_AMR-Policy-Update-5.png)