[HGPI Policy Column] No. 33 – From the NCDs (Precision Cancer Medicine) Team, What is precision cancer medicine?
date : 2/2/2023
![[HGPI Policy Column] No. 33 – From the NCDs (Precision Cancer Medicine) Team, What is precision cancer medicine?](https://hgpi.org/en/wp-content/uploads/sites/2/column-33-top.png)
1. In treating cancer, there are three major therapies, namely, (i) Surgical operation, (ii) Radiation therapy, and (iii) Drug therapy (Chemotherapy). Recently, treatment options have been increasing—for example, (iv) Cancer immunotherapy, which uses treatments to stimulate or restore the ability of the immune system to fight cancer, has become one of the major treatment options (see table below).
“Precision cancer medicine,” which has been attracting attention in recent years, is particularly related to (iii) Drug therapy. They identify the mutated genes, cancer cells of individual patients to be treated in advance, and then, “molecular-targeted drugs” (drugs that are particularly effective on those mutated genes and molecules) are selected and administered.
In conventional cancer drug therapy, cancers are usually perceived in terms of the affected organ, and drugs are administered with the expectation they will be effective for that type of cancer. In precision cancer medicine, they provide treatment in a more targeted manner, by focusing on the section of the gene where mutations are occurring. This is why it is called “precision” medicine.
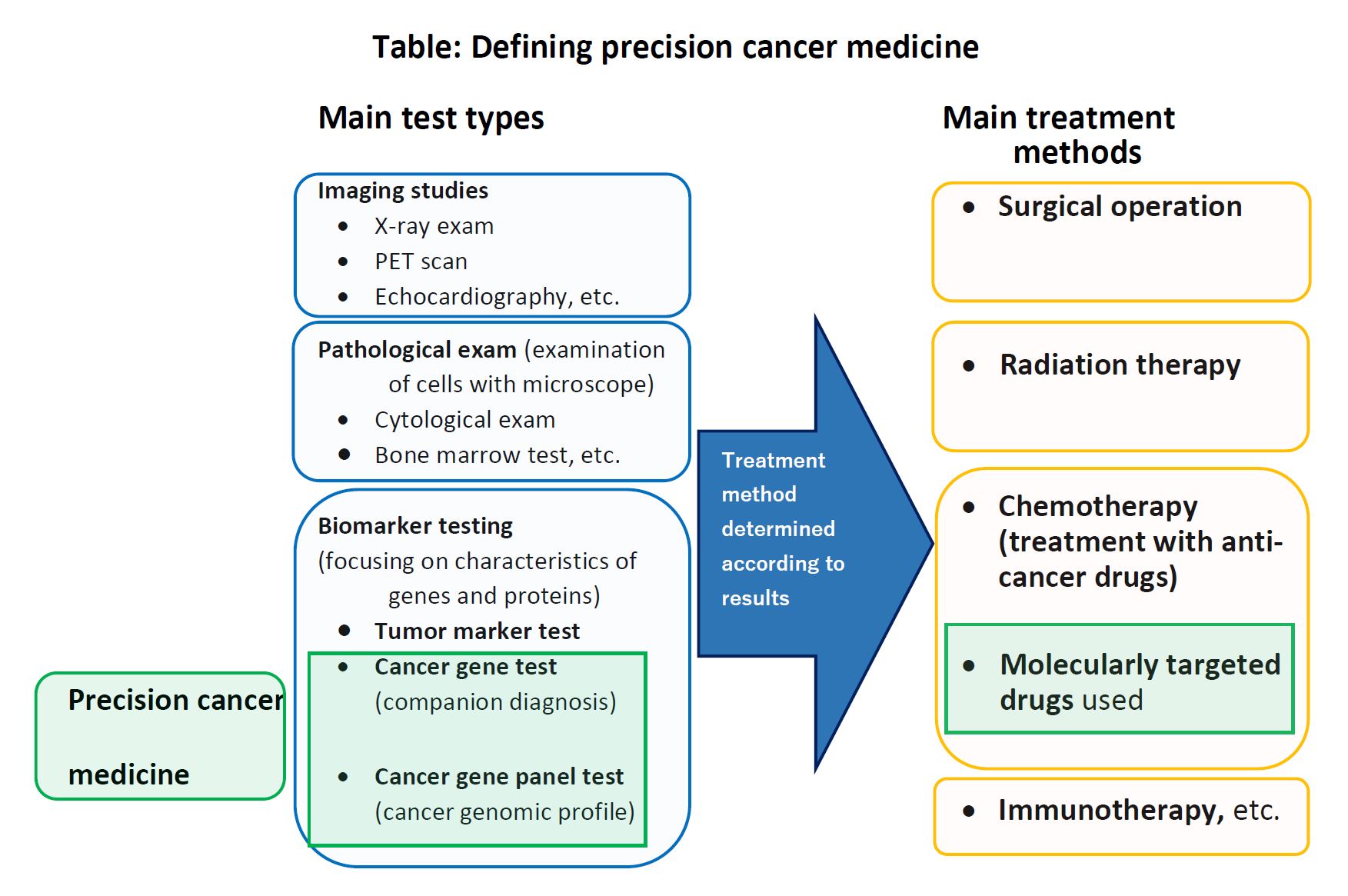
2. To carry out precision cancer medicine, genetic testing is indispensable. There are two methods of genetic testing for cancer: (i) “Cancer gene testing “or “Companion diagnostic testing” and (ii) “Cancer gene panel testing” or “Cancer genome profiling test.” Cancer gene testing determines the presence or absence of mutations in one or several genes, in order to determine in advance whether a certain therapeutic drug will be effective for a patient’s cancer. Cancer gene panel testing is to determine the presence or absence of mutations in a large number of genes (usually more than one hundred) at once.
3. Compared to conventional cancer drug therapy, more targeted therapies are administered in precision cancer medicine, and this can reduce burdens on patients, increase response rates, and achieve better outcomes. There are high expectations for the future development of precision cancer medicine.
On the other hand, there are many challenges: (a) the costs of testing and treatment are often quite expensive; (b) even if a genetic testing is conducted, it may not find a genetic mutation that is eligible for precision cancer medicine (in such a case, conventional drug therapy is an option); (c) nationwide, specialized facilities and human resources to conduct genetic testing and treatment are not that abundant; and (d) laws and regulations to strictly manage genetic information and to prevent genetic discrimination have not yet been fully established.
In order to further develop precision cancer medicine in the future, it is necessary to resolve these various issues as soon as possible.
Column authors
- Sayaka Honda (Program Specialist, HGPI)
- Osamu Takemoto (Program Specialist, HGPI)
- Haruka Sakamoto (Senior Manager, HGPI)
HGPI Policy Column (No.32) -From thePlanetary Health Policy Team- >
Top Research & Recommendations Posts
- [Research Report] Perceptions, Knowledge, Actions and Perspectives of Healthcare Organizations in Japan in Relation to Climate Change and Health: A Cross-Sectional Study (November 13, 2025)
- [Policy Recommendations] Reshaping Japan’s Immunization Policy for Life Course Coverage and Vaccine Equity: Challenges and Prospects for an Era of Prevention and Health Promotion (April 25, 2025)
- [Research Report] The 2025 Public Opinion Survey on Healthcare in Japan (March 17, 2025)
- [Research Report] The 2026 Public Opinion Survey on Healthcare in Japan (February 13, 2026)
- [Research Report] 2019 Survey on Healthcare in Japan
- [Research Report] Building a Mental Health Program for Children and Measuring its Effectiveness (June 16, 2022)
- [Policy Recommendations] Developing a National Health and Climate Strategy for Japan (June 26, 2024)
- [Research Report] The Public Opinion Survey on Child-Rearing in Modern Japan (Final Report) (March 4, 2022)
- [Research Report] The 2023 Public Opinion Survey on Satisfaction in Healthcare in Japan and Healthcare Applications of Generative AI (January 11, 2024)
- [Policy Recommendations] Mental Health Project: Recommendations on Three Issues in the Area of Mental Health (July 4, 2025)
Featured Posts
-
2026-01-09
[Registration Open] (Hybrid Format) Dementia Project FY2025 Initiative Concluding Symposium “The Future of Dementia Policy Surrounding Families and Others Who Care for People with Dementia” (March 9, 2026)
![[Registration Open] (Hybrid Format) Dementia Project FY2025 Initiative Concluding Symposium “The Future of Dementia Policy Surrounding Families and Others Who Care for People with Dementia” (March 9, 2026)](https://hgpi.org/en/wp-content/uploads/sites/2/dementia-20260309-top.png)
-
2026-02-27
[Registration Open] (Webinar) The 142nd HGPI Seminar “World Kidney Day 2026” Theme in Focus: The Current State of Green Nephrology and Green Dialysis—Balancing Kidney Health and Planetary Health (March 10, 2026)
![[Registration Open] (Webinar) The 142nd HGPI Seminar “World Kidney Day 2026” Theme in Focus: The Current State of Green Nephrology and Green Dialysis—Balancing Kidney Health and Planetary Health (March 10, 2026)](https://hgpi.org/en/wp-content/uploads/sites/2/The142nd_HGPI_Seminar.jpg)
-
2026-03-03
[Registration Open] Online Seminar “Implementing Chronic Kidney Disease (CKD) Measures into Society: Towards a Data-Driven Health System” (April 21, 2026)
![[Registration Open] Online Seminar “Implementing Chronic Kidney Disease (CKD) Measures into Society: Towards a Data-Driven Health System” (April 21, 2026)](https://hgpi.org/en/wp-content/uploads/sites/2/HGPI_20260421_CKDonlineseminar-.png)




