[Policy Recommendations] Improving Patient Access to Genomic Cancer Medicine (August 10, 2023)
date : 8/10/2023
Tags: NCDs, Precision Cancer Medicine
![[Policy Recommendations] Improving Patient Access to Genomic Cancer Medicine (August 10, 2023)](https://hgpi.org/en/wp-content/uploads/sites/2/ncd-20230810-figure1_ENG.jpg)
Health and Global Policy Institute (HGPI) has presented a policy recommendation paper regarding the improvement of patient access to Genomic Cancer Medicine.
Executive summary
- Genomic Cancer Medicine was viewed as an advanced form of medical treatment when Comprehensive Genomic Profiling (CGP; or cancer gene panel tests) was granted health insurance coverage in 2019. Since then, people serving in clinical settings have accumulated experience handling this form of treatment. We are now at a stage to make Genomic Cancer Medicine a form of technology from which anyone should be able to benefit. To achieve this, we should improve patient access to deliver Genomic Cancer Medicine more broadly to the public.
- From the perspective of patient access opportunities, policy challenges with respect to delivering Genomic Cancer Medicine may be sorted into three categories:
A: Constraints related to human resources in medicine
B: Constraints related to genetic testing
C: Constraints related to geographic factors or information
We must take wherever possible actions to address each issue one by one, and expand opportunities for patients to access Genomic Cancer Medicine.
- Based on these understandings, HGPI offers the following recommendations:
RECOMMENDATION I: Streamline operations and secure human resources
In view of the further spread of genomic cancer medicine, streamline operations thoroughly, and adopt systems to secure human resources commensurate with workloads.
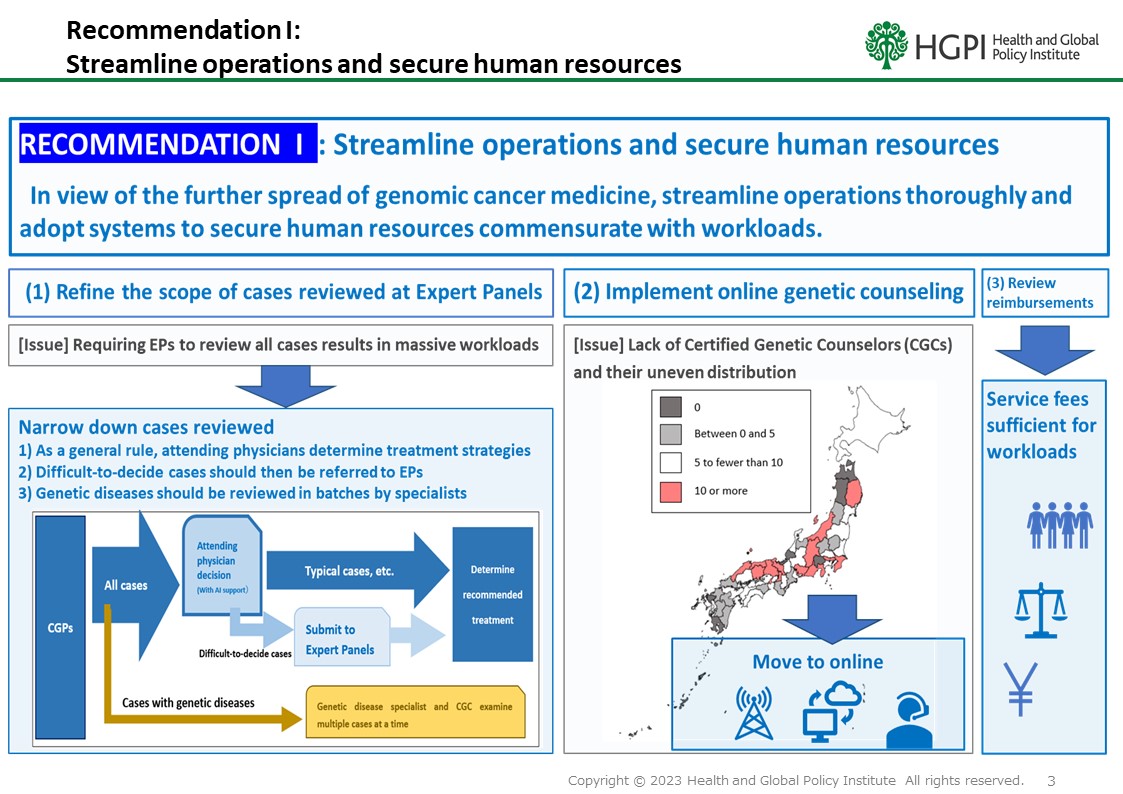
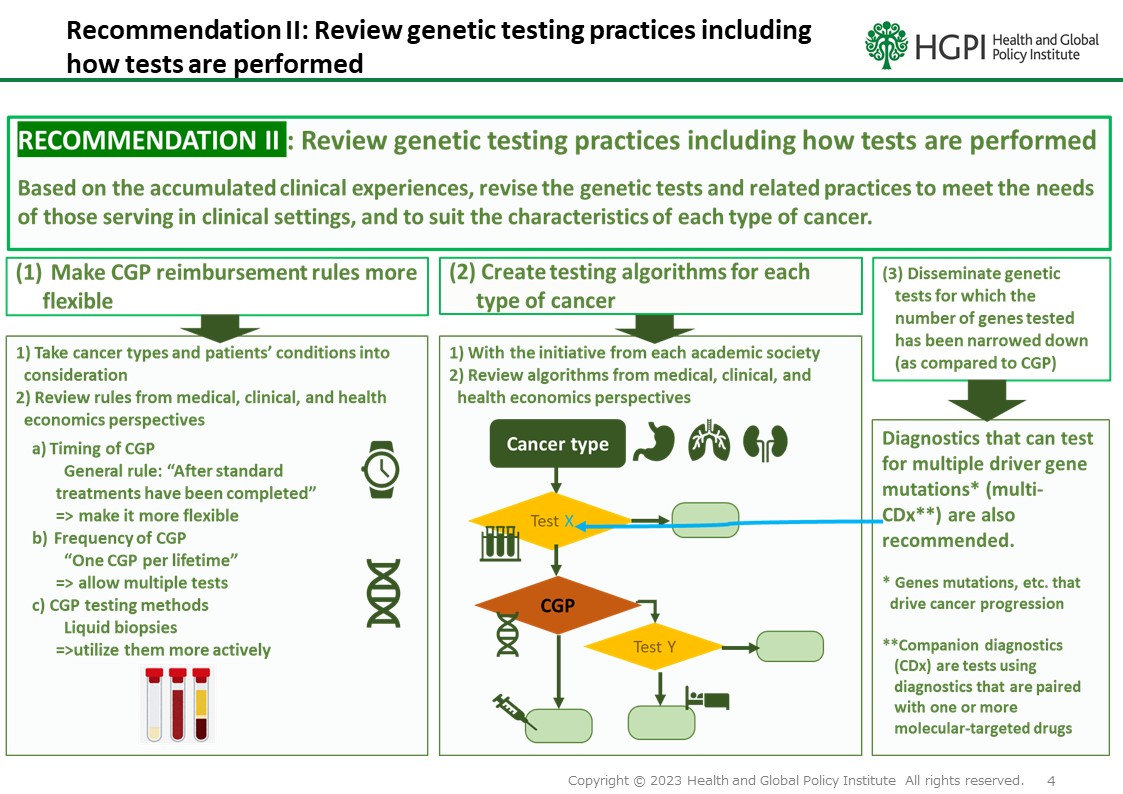
RECOMMENDATION II: Review genetic testing practices including how tests are performed
Based on the accumulated clinical experiences, revise the genetic tests and related practices to meet the needs of those serving in clinical settings, and to suit the characteristics of each type of cancer.
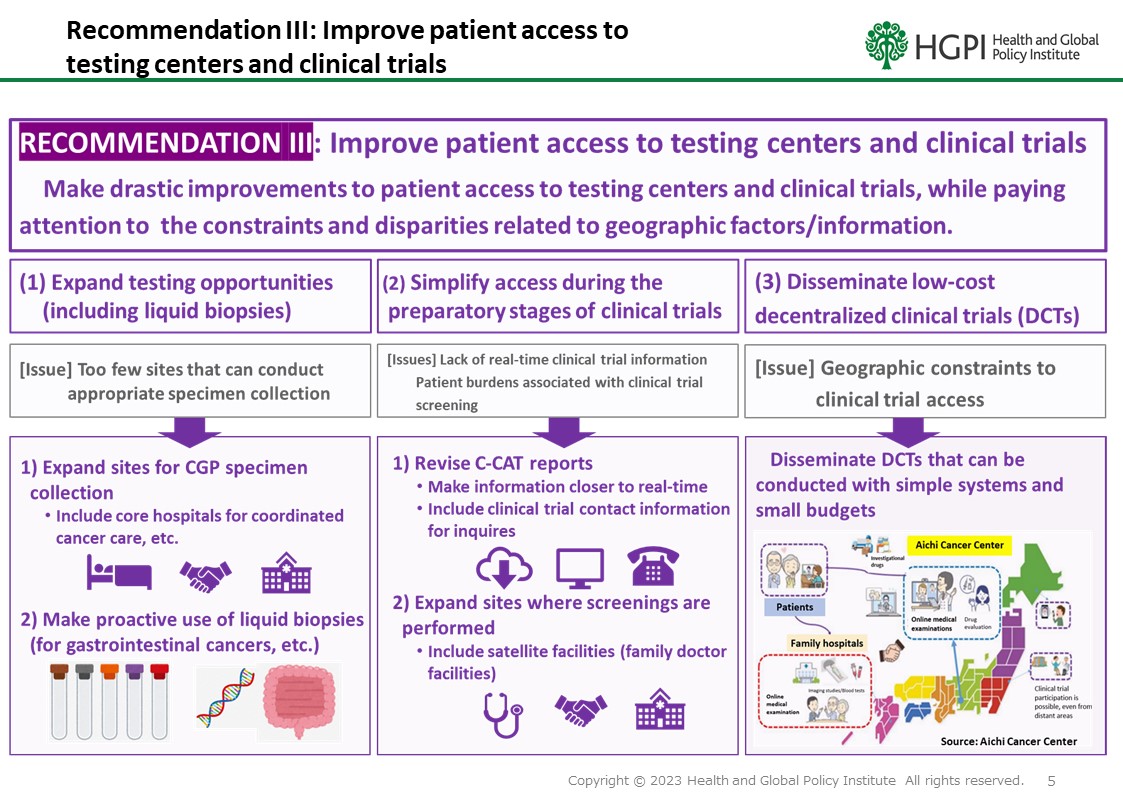
RECOMMENDATION III: Improve patient access to testing centers and clinical trials
While paying attention to the constraints and disparities related to geographic factors/information, make drastic improvements to patient access to testing centers and clinical trials.
As for precision cancer medicine, HGPI launched an initiative in FY2021 (“Project for Considering the Future of Precision Medicine with Industry, Government, Academia, and Civil Society”), and has made expert hearings and conducted surveys to examine the issue. Based on our findings, we offered comprehensive policy recommendations in September 2022.
For the second phase of this project, we compiled this policy recommendation paper, based on additional, expansive surveys and hearings with our focus on measures for better patient access to Genomic Cancer Medicine.
HGPI strongly hopes these recommendations will be utilized in developing further policy measures for Genomic Cancer Medicine, and patient-centered healthcare.
For details, please see the PDFs linked below.
Sponsors (in alphabetical order):
Bayer Yakuhin, Ltd.
Chugai Pharmaceutical Co., Ltd.
Janssen Pharmaceutical K.K.
MSD K.K.
Top Research & Recommendations Posts
- [Policy Recommendations] The Path to a Sustainable Healthcare System: Three Key Objectives for Public Deliberation (January 22, 2026)
- [Research Report] The 2025 Public Opinion Survey on Healthcare in Japan (March 17, 2025)
- [Research Report] Perceptions, Knowledge, Actions and Perspectives of Healthcare Organizations in Japan in Relation to Climate Change and Health: A Cross-Sectional Study (November 13, 2025)
- [Policy Recommendations] Reshaping Japan’s Immunization Policy for Life Course Coverage and Vaccine Equity: Challenges and Prospects for an Era of Prevention and Health Promotion (April 25, 2025)
- [Research Report] The 2023 Public Opinion Survey on Satisfaction in Healthcare in Japan and Healthcare Applications of Generative AI (January 11, 2024)
- [Research Report] AMR Policy Update #4: Cancer Care and AMR (Part 1)
- [Public Comment Submission] “Assessment Report on Climate Change Impacts in Japan (Draft Overview)” (December 24, 2025)
- [Policy Recommendations] Developing a National Health and Climate Strategy for Japan (June 26, 2024)
- [Research Report] The Public Opinion Survey on Child-Rearing in Modern Japan (Final Report) (March 4, 2022)
- [Research Report] Survey of Japanese Physicians Regarding Climate Change and Health (December 3, 2023)
Featured Posts
-
2026-01-09
[Registration Open] (Hybrid Format) Dementia Project FY2025 Initiative Concluding Symposium “The Future of Dementia Policy Surrounding Families and Others Who Care for People with Dementia” (March 9, 2026)
![[Registration Open] (Hybrid Format) Dementia Project FY2025 Initiative Concluding Symposium “The Future of Dementia Policy Surrounding Families and Others Who Care for People with Dementia” (March 9, 2026)](https://hgpi.org/en/wp-content/uploads/sites/2/dementia-20260309-top.png)
-
2026-02-05
[Registration Open] (Webinar) The 141st HGPI Seminar “Current Status and Future Prospects of Korea’s Obesity Policy: Voices of People with Lived Experience in Policy Promotion” (March 3, 2026)
![[Registration Open] (Webinar) The 141st HGPI Seminar “Current Status and Future Prospects of Korea’s Obesity Policy: Voices of People with Lived Experience in Policy Promotion” (March 3, 2026)](https://hgpi.org/en/wp-content/uploads/sites/2/hs141-top-1.png)
-
2026-02-06
[Research Report] AMR Policy Update #5: Cancer Care and AMR (Part 2)
![[Research Report] AMR Policy Update #5: Cancer Care and AMR (Part 2)](https://hgpi.org/en/wp-content/uploads/sites/2/HGPI_20260204_AMR-Policy-Update-5.png)