[Research Report] Results of Internet Survey on Genomic Cancer Medicine (Summary)(May 11, 2023)
date : 6/12/2023
Tags: NCDs, Precision Cancer Medicine
![[Research Report] Results of Internet Survey on Genomic Cancer Medicine (Summary)(May 11, 2023)](https://hgpi.org/en/wp-content/uploads/sites/2/Genomic-Cancer-Medicine_230511-1.png)
The English version of the report has been published. (June 12, 2023)
In March 2023, Health and Global Policy Institute (HGPI) Project for Considering the Future of Precision Medicine with Industry, Government, Academia, and Civil Society and the Section of Global Health of the Tokyo Women’s Medical University Department of Hygiene and Public Health conducted an online survey among cancer patients and their families to assess the current status of genomic cancer medicine. Topics it examined included (1) awareness toward genomic cancer medicine, (2) awareness toward cancer gene panel testing, and (3) access to cancer gene panel testing.
●Key Survey Findings
- Among individuals who have been diagnosed with cancer or have had a family member who was diagnosed with cancer within the past five years, 40% of respondents had heard of genomic cancer medicine.
- Compared to other age groups, respondents in the group age 60 years and older were less likely to have heard of “genomic cancer medicine” or “cancer gene panel testing,” or to have received explanations about genomic cancer medicine from physicians.
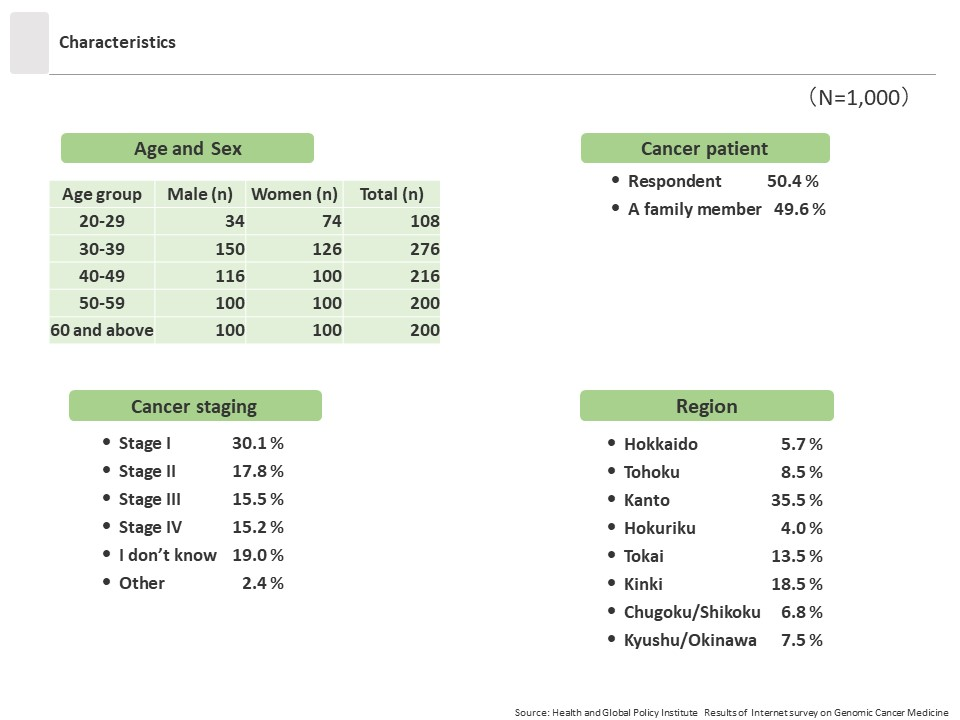
[Survey Overview]
This survey was conducted in late March 2023 using an online survey targeting 1,000 men and women age 20 years or older who had been diagnosed with cancer or who had family members who had been diagnosed with cancer. The survey was administered after its purpose was explained and respondent consent was obtained.
- Research Period: Late March 2023
- Method: Internet survey
- Subjects: Men and women age 20 years or older in Japan registered in the disease panel of the research firm Cross Marketing Inc. Registrants to this disease panel are those who had visited a hospital for cancer within the past year. Among them, the target participants of this survey were those who had either been diagnosed with cancer or who had a family member who had been diagnosed with cancer within the past five years. The total sample size was 1,000 respondents, with 500 men and 500 women.
- Number of responses: 1,000
●Results
1. Awareness toward “genomic cancer medicine” and related topics
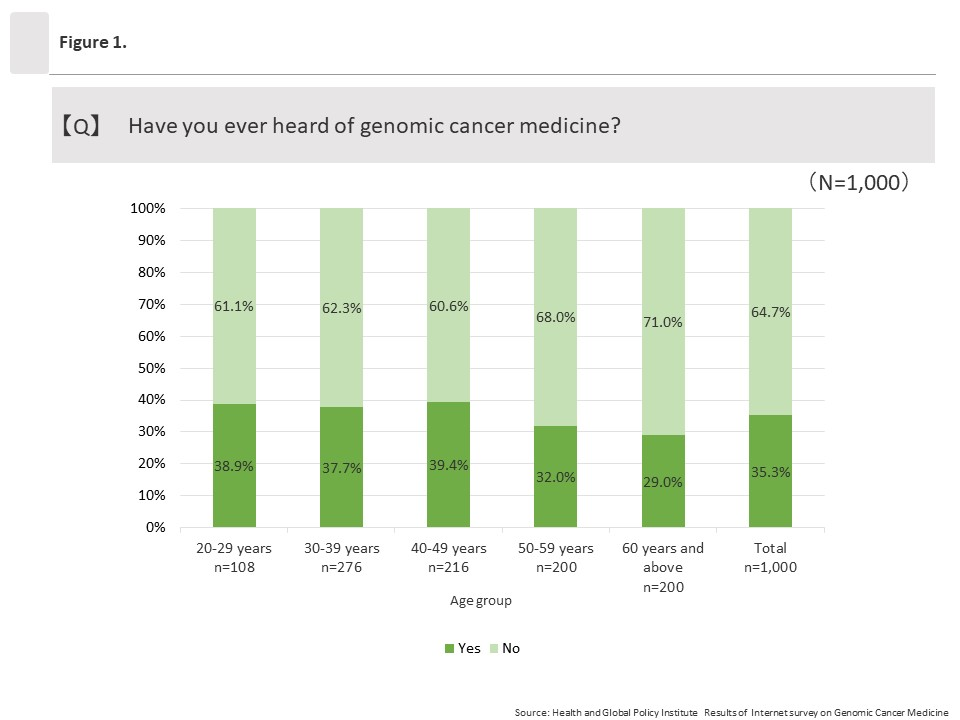
(1) Awareness toward “genomic cancer medicine”
The overall percentage of respondents who answered “Yes” to the question “Have you ever heard of genomic cancer medicine?” was 35.3% (353/1,000). By age group, approximately 40% of respondents in the groups ages 20 to 29 and 40 to 49 had heard of genomic cancer medicine compared to about 30% in the groups ages 50 to 59 and 60 and above.
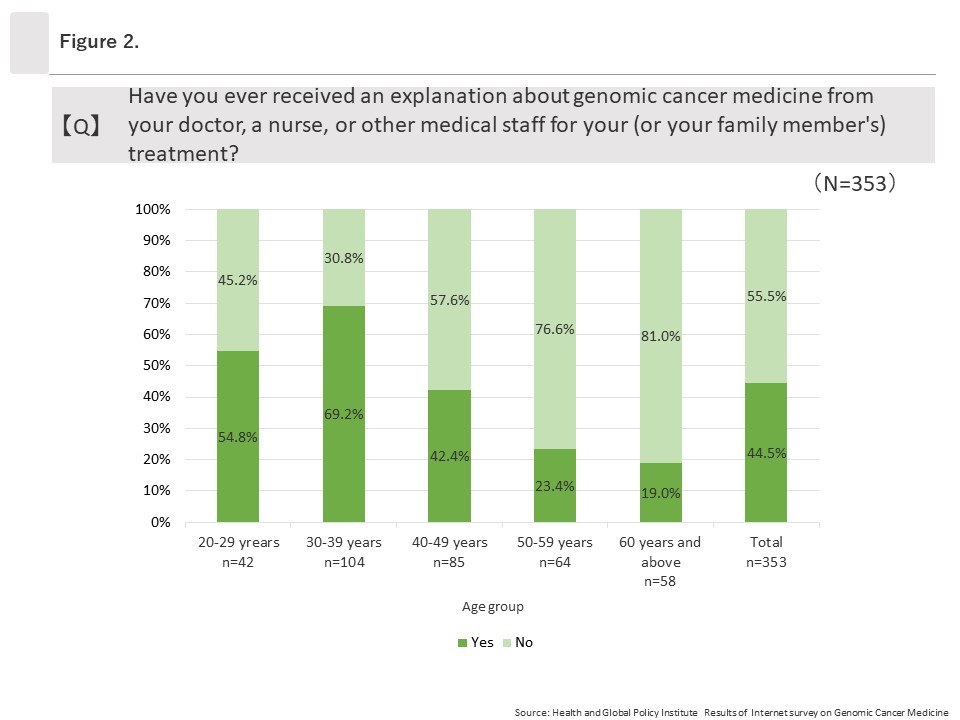
(2) Explanations regarding “genomic cancer medicine” from physicians or other healthcare professionals
Among the 353 respondents who had heard of genomic cancer medicine, 44.5% (157/353) answered “Yes” when asked, “Have you ever received an explanation about genomic cancer medicine from your doctor, a nurse, or other medical staff for your (or your family member’s) treatment?” Looking at “Yes” responses by age group, the group ages 30 to 39 had the highest rate at 69.2% (72/104) and affirmative response rates decreased as ages increased. The lowest rate was among those ages 60 and above, at 19.0% (11/58).
2. Awareness toward “cancer gene panel testing (genomic cancer profiling)” and related topics
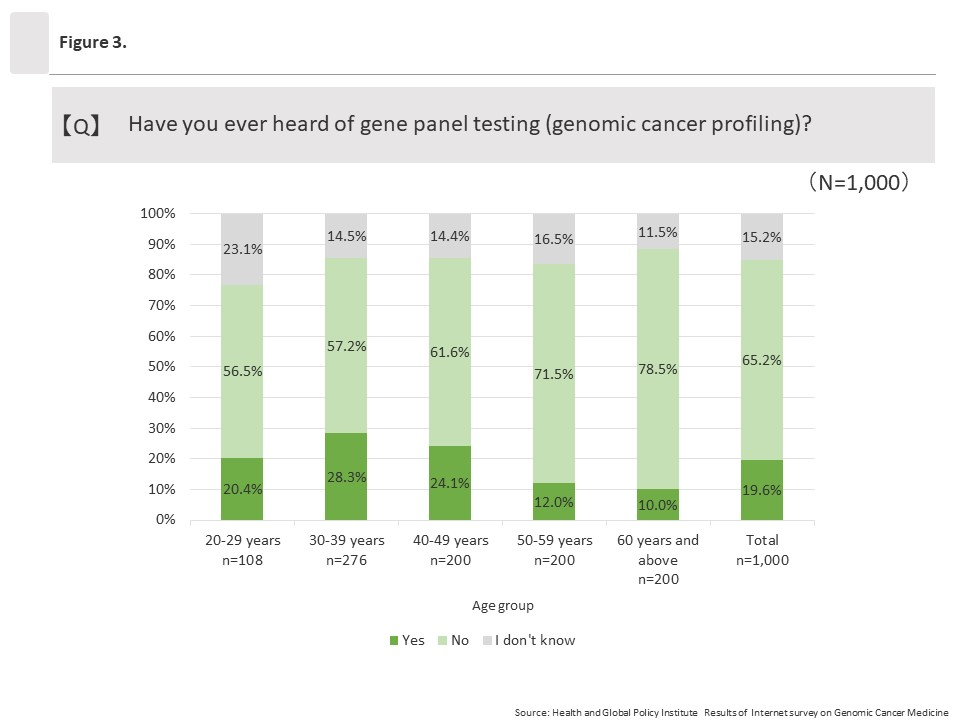
(1) Awareness toward “cancer gene panel testing (genomic cancer profiling)”
Among all respondents, 19.6% (196/1,000) said they had heard of cancer gene panel testing (genomic cancer profiling). By age group, those ages 30 to 39 answered “Yes” at the highest rate at 28.3% (78/276), and affirmative response rates decreased as ages increased. The lowest rate was among those ages 60 and above, at 10.0% (20/200).
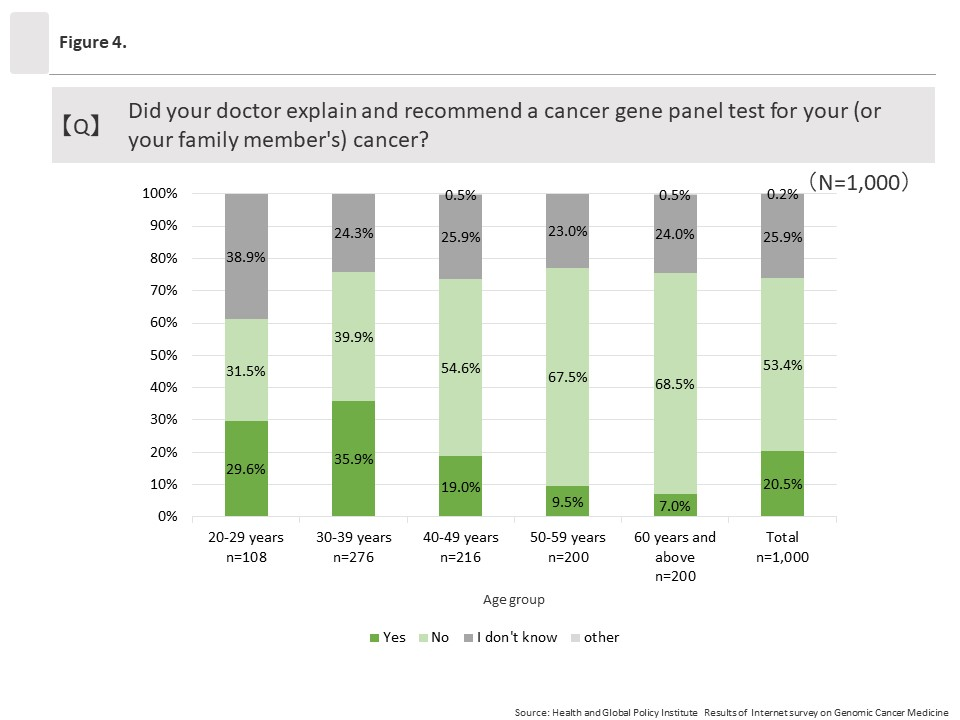
(2) Explanations from doctors regarding “cancer gene panel testing”
1. Regarding all responses to “Did your doctor explain and recommend a cancer gene panel test for your (or your family member’s) cancer?”, 20.5% (205/1,000) said “Yes;” 53.4% (534/1,000) said “No;” and 25.9% (259/1,000) responded “I don’t know.”
2. When the 205 respondents who said “Yes” were asked when they received such explanations, most (96/205) said they received explanations “Before standard treatment,” followed by “During standard treatment” (84/205) and “After standard treatment” (25/205).
3. Timeframes for cancer gene panel testing and related topics
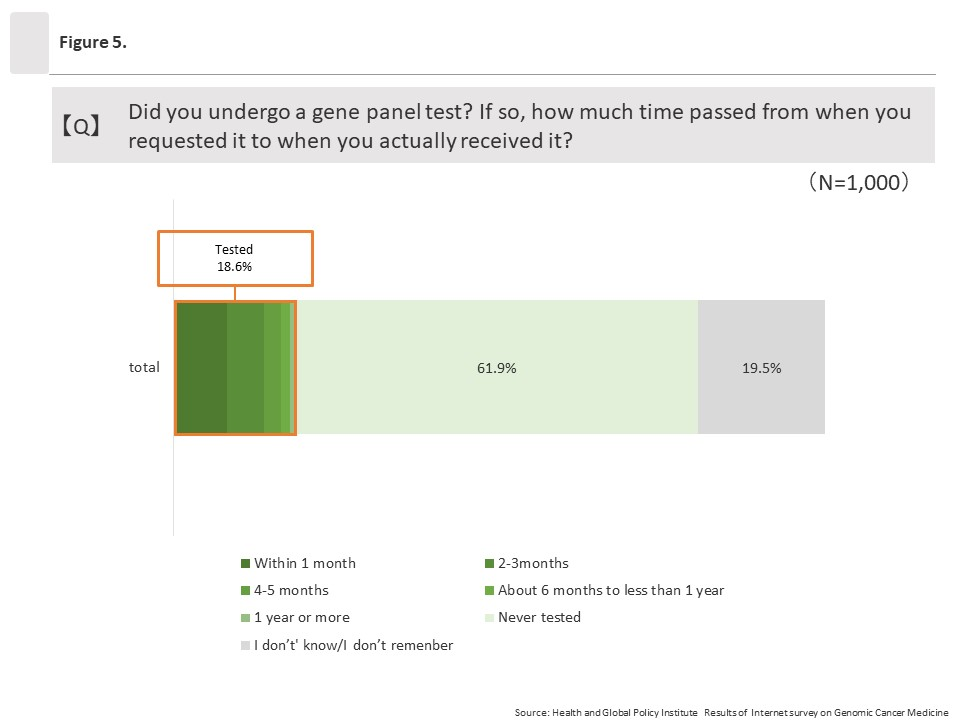
(1) Percentage of respondents who underwent cancer gene panel testing
The overall percentage of respondents who answered “Yes” to the question “Did you undergo a gene panel test?” was 18.6% (186/1,000).
(2) Number of days required to receive cancer gene panel tests and test location
Among the respondents who had taken cancer gene panel tests (186/1,000), almost half (81/186) said they were able to take the test within one month from the time it was requested.
Regarding the location of the test, among 186 respondents who underwent cancer gene panel testing, 71 respondents answered, “A medical institution in the municipality where I live;” 72 said, “A medical institution in the same prefecture where I live, but in a different municipality;” 31 selected, “A medical institution in a neighboring prefecture (outside of the prefecture where I live);” and 12 said, “A distant prefecture other than neighboring prefectures.”
4. Limitations and Prospects
There were three limitations to this survey.
First, this survey was conducted using the Internet among panel registrants, which may have introduced sampling bias. Respondents were limited to those who have access to the Internet, there may have been biases related to respondents’ educational backgrounds,[1] and the survey sample might not have been nationally representative.
Second, the number of responses was limited. While the sample size provides some insights, it may not be large enough for certain analyses or subgroup analysis. Results should be interpreted with caution, especially when stratifying the data.
Third, the results presented in the survey report are cross-tabulations, and no tests of statistical significance were conducted.
Survey results are planned to be presented in a peer-reviewed paper that will be published jointly with the Section of Global Health of the Tokyo Women’s Medical University Department of Hygiene and Public Health, which also participated in conducting this survey.
[1] Hanibuchi N, Muranaka A, Ando M, 2015, Challenges of Data Collection through Internet Research: Analysis of “Frivolous” Responses, Response Time, and Geographical Pattern. E-journal GEO, 10 (1), 81-98.
Top Research & Recommendations Posts
- [Policy Recommendations] Reshaping Japan’s Immunization Policy for Life Course Coverage and Vaccine Equity: Challenges and Prospects for an Era of Prevention and Health Promotion (April 25, 2025)
- [Research Report] Perceptions, Knowledge, Actions and Perspectives of Healthcare Organizations in Japan in Relation to Climate Change and Health: A Cross-Sectional Study (November 13, 2025)
- [Research Report] The 2025 Public Opinion Survey on Healthcare in Japan (March 17, 2025)
- [Research Report] The 2026 Public Opinion Survey on Healthcare in Japan (February 13, 2026)
- [Research Report] 2019 Survey on Healthcare in Japan
- [Research Report] Building a Mental Health Program for Children and Measuring its Effectiveness (June 16, 2022)
- [Policy Recommendations] Developing a National Health and Climate Strategy for Japan (June 26, 2024)
- [Research Report] The Public Opinion Survey on Child-Rearing in Modern Japan (Final Report) (March 4, 2022)
- [Research Report] The 2023 Public Opinion Survey on Satisfaction in Healthcare in Japan and Healthcare Applications of Generative AI (January 11, 2024)
- [Policy Recommendations] Mental Health Project: Recommendations on Three Issues in the Area of Mental Health (July 4, 2025)
Featured Posts
-
2026-01-09
[Registration Open] (Hybrid Format) Dementia Project FY2025 Initiative Concluding Symposium “The Future of Dementia Policy Surrounding Families and Others Who Care for People with Dementia” (March 9, 2026)
![[Registration Open] (Hybrid Format) Dementia Project FY2025 Initiative Concluding Symposium “The Future of Dementia Policy Surrounding Families and Others Who Care for People with Dementia” (March 9, 2026)](https://hgpi.org/en/wp-content/uploads/sites/2/dementia-20260309-top.png)
-
2026-02-27
[Registration Open] (Webinar) The 142nd HGPI Seminar “World Kidney Day 2026” Theme in Focus: The Current State of Green Nephrology and Green Dialysis—Balancing Kidney Health and Planetary Health (March 10, 2026)
![[Registration Open] (Webinar) The 142nd HGPI Seminar “World Kidney Day 2026” Theme in Focus: The Current State of Green Nephrology and Green Dialysis—Balancing Kidney Health and Planetary Health (March 10, 2026)](https://hgpi.org/en/wp-content/uploads/sites/2/The142nd_HGPI_Seminar.jpg)
-
2026-03-03
[Registration Open] Online Seminar “Implementing Chronic Kidney Disease (CKD) Measures into Society: Towards a Data-Driven Health System” (April 21, 2026)
![[Registration Open] Online Seminar “Implementing Chronic Kidney Disease (CKD) Measures into Society: Towards a Data-Driven Health System” (April 21, 2026)](https://hgpi.org/en/wp-content/uploads/sites/2/HGPI_20260421_CKDonlineseminar-.png)