[Event Report] The 113th HGPI Seminar – Current Circumstances and Issues in Precision (Genomic) Cancer Medicine (March 2, 2023)
date : 4/12/2023
![[Event Report] The 113th HGPI Seminar – Current Circumstances and Issues in Precision (Genomic) Cancer Medicine (March 2, 2023)](https://hgpi.org/en/wp-content/uploads/sites/2/hs113-top_JPNENG-1.png)
For the 113th HGPI Seminar, we hosted Dr. Atsushi Otsu, Hospital Director of National Cancer Center Hospital East. Dr. Otsu gave a comprehensive lecture on a number of subjects including current circumstances, issues, and latest developments in R&D in the field of precision cancer medicine.
Key points of the lecture
- In precision or genomic cancer medicine, gene panel tests and other genetic analyses are conducted and patients are administered the optimal drug therapies based on the findings of those analyses. While this form of treatment has been gradually spreading in Japan over the past few years, it is difficult to say it has become sufficiently widespread.
- There are various factors that have led to the insufficient dissemination of precision cancer medicine. They include (1) insufficient human resources and facilities specializing in precision cancer medicine; (2) restrictions on the timing of gene panel tests and how many can be administered; (3) heavy workloads for the expert panels that assess gene panel test results; and (4) the low probability that effective treatments will be found in the event that gene alterations like mutations, amplification, and fusions are detected.
- Increasing the number of people enrolled in clinical trials will be vital to increase the number of drugs that are approved (as well as to increase treatment methods that match gene alterations). To help do so, (1) National Cancer Center Hospital East is leading efforts to build a system that will allow physicians to share clinical trial information in a more timely manner; and (2) a platform for genomic medicine development called SCRUM-Japan is being utilized to streamline clinical trials, which is improving efficiency in the approval process. Expectations are also growing for (3) Decentralized Clinical Trials (DCTs), which will allow people living in rural areas to participate in clinical trials online, with no need to visit trial sites.
- Liquid biopsies are a type of blood test that is used to detect gene alterations in cancer cells and that are becoming more widely used. More widespread use of liquid biopsies has reduced physical and mental burdens on patients while enabling faster turnaround time (TAT), or the time from when tests are conducted to when treatments begin. They have also led to significant growth in clinical trial enrollment. There are also high expectations for liquid biopsies to be used to detect minimal residual disease (MRD) through circulating tumor DNA (ctDNA; cancer cell DNA that has leaked into the bloodstream due to causes like cell death) to predict cancer recurrence.
- Even though advances have been made in genome analysis, we are unable to discover many gene alterations that cause cancer to progress, which are known as driver genes. There have also been a series of successes in drug discovery in recent years, such as in the development of antibody-drug conjugates (ADCs), radiopharmaceuticals, and middle molecule drugs. This emphasis on the drug discovery environment is driving a shift toward an approach in which therapeutic drug targets are identified by analyzing a broad range of information including RNA and proteins (known as “multi-omics analysis”).
Precision cancer (genomic) medicine is the administration of optimal drug therapies based on genetic analyses
“Precision cancer medicine” or “genomic medicine” refer to “Examining the genes in tissues from a patient and administering suitable therapeutics.” A number of gene alterations that cause cancer to progress or “driver genes” have been identified. Compared to patients without driver genes, or to patients with driver genes but who are not administered drugs that match their gene alterations, survival rates have improved for patients with driver genes and who are administered molecularly targeted drugs.
In addition to forms of genetic testing like gene panel tests (or, cancer genomic profiling), in which hundreds of genes are analyzed simultaneously, recent years have seen the widespread and rapid adoption of liquid biopsies, which are used to examine cancer cell DNA that has leaked into the bloodstream.
Precision cancer medicine has not spread enough
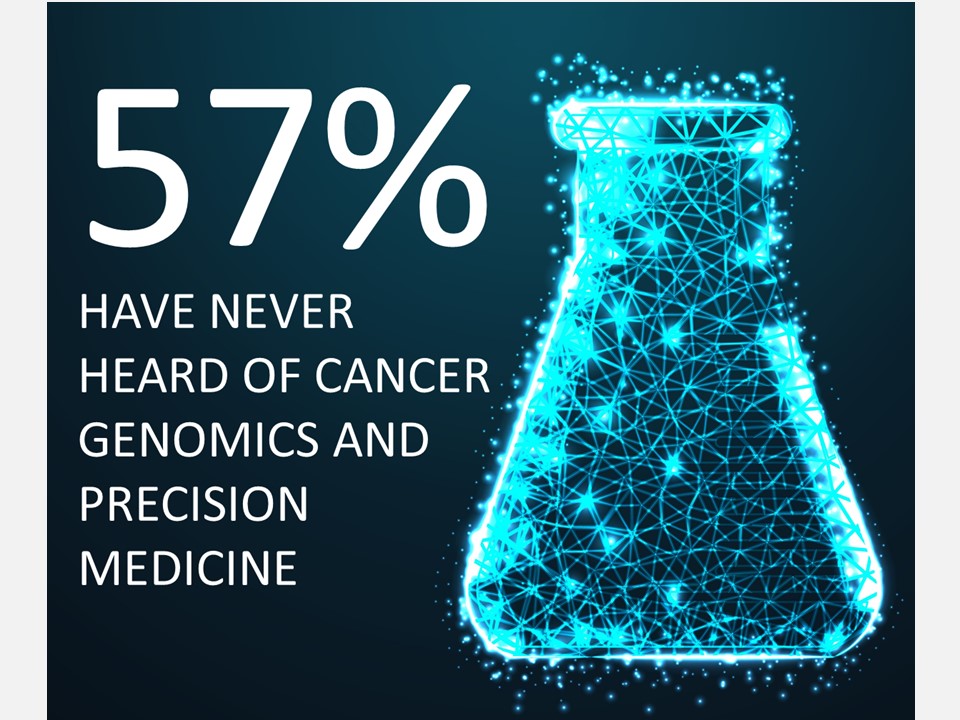
After gene panel tests were granted insurance coverage in 2019, precision cancer medicine grew more widespread in Japan, but that growth has not been sufficient. Among the 380,000 people who died of cancer in FY2021, over half of them were likely to have been candidates for precision cancer medicine. However, only around 14,000 cancer genome tests were actually performed that year.
There are multiple issues that must be addressed to disseminate precision cancer medicine
There are a number of factors that have led to insufficient growth in the use of precision cancer medicine, but key factors are described below.
(1) The number of physicians with the expertise to conduct testing and the number of facilities with the capacity to provide such tests are limited. (Testing can only be performed at central and core cancer hospitals for genomic cancer treatment or core hospitals for coordinated genomic cancer treatment. Combined, there are a total of 243 such facilities[1] as of 6 March.)
(2)After completion of standard treatment (or when standard treatment is expected to complete), the number of tests that can be performed over a patient’s lifetime is limited to one. In addition to these high restrictions, in practice, it is also difficult to wait several weeks for test results when a patient’s condition is growing worse.
(3) Conducting the expert panels that evaluate gene panel test results places extreme workloads on the personnel at the central and core cancer hospitals for genomic cancer treatment who are in charge of doing so.
(4) Because driver genes that have suitable molecularly targeted drugs are still few in number, even when a gene panel test is conducted, there is a low probability that approved drugs, suitable clinical trials, or other treatments that match the gene alteration in question will be found. This discourages clinicians from attempting to provide precision cancer medicine to cancer patients.
(5) Clinical data from people who receive precision cancer medicine must be registered with the Center for Cancer Genomics and Advanced Therapeutics (C-CAT),[2] but the data entry required for the registration process poses a heavy workload.
Precision cancer medicine is not yet a complete form of medicine
As described above, even when a gene panel test is performed, the chances of the patient arriving at a compatible therapeutic agent are currently very low. Furthermore, even if two genes have the same alteration, there are times when a drug that is effective for one type of cancer is not as effective for another type. This means that the efficacy of each treatment must be confirmed by driver gene or by organ through clinical trials. As such, it cannot be said that precision cancer medicine is a complete form of medicine.
Clinical trials will be vital for delivering precision cancer medicine to patients
Other than treatments covered by health insurance, people have three options for receiving precision cancer medicine: (1) clinical trials; (2) Patient-Proposed Healthcare Services programs (such as NCCH1901); and (3) off-label use (which is the use of an approved drug for unapproved indications, or the use of unapproved dosages or methods of administration).
Regarding (1) clinical trials, the subjects are not required to bear any of the cost burden, and if the trial obtains good results, the therapeutic agent will be approved by regulatory bodies and granted insurance coverage so it can be used nationwide. However, for (2) Patient-Proposed Healthcare Services, the patient also bears none of the cost burden when the therapeutic agent is supplied by a company, but there is an issue in that it is unclear whether data will lead to regulatory approval afterward. Finally, for (3) off-label use, the patient must import the therapeutic agent on their own and must bear a high cost burden. Additionally, in the majority of cases, there is insufficient evidence for such treatments even overseas, so there are many times when the treatments are ineffective and patients experience only side effects.
Considering these points, we can reaffirm that increasing the number of domestic clinical trials, enrolling more patients, and obtaining more approvals will be vital for delivering precision cancer medicine to a greater number of people in Japan.
Establishing a framework for improving clinical trial enrollment will be vital
It has been reported that clinical trial enrollment improved when sufficient information regarding the kinds of trials that are being conducted and what kinds of facilities are being used to conduct them was shared among responsible parties on expert panels. Based on such research findings, the National Cancer Center Hospital East is currently leading efforts to build an organization called the “Academic Assembly” which will serve to share clinical trial-related information in a more timely manner as well as match subjects to trials.
In addition to this, one noteworthy global trend is the spread of Decentralized Clinical Trials (DCTs). Conventional clinical trials faced the limitation that participants had to visit clinical trial sites in order to participate, even if they lived far away. With DCTs, however, it is possible for people to participate remotely, such as by using digital devices to access online medical services or by having therapeutic agents brought to them using delivery services. Because DCTs make it possible for subjects to participate in clinical trials without having to go to the trouble of visiting a hospital, they have the potential to eliminate regional disparities in clinical trials.
Streamlining clinical trials with SCRUM-Japan, a platform for genomic medicine development
In the areas of drug discovery and R&D in precision medicine, a platform called SCRUM-Japan[3] was established in 2015 with the objective of developing effective therapeutic agents and delivering them to patients as quickly as possible. Its registrants currently include 210 academic facilities and they are jointly advancing many clinical trials with 18 pharmaceutical companies. Registrants share an online clinical genome database with over 40,000 samples. Efforts made through SCRUM-Japan have resulted in a number of papers on drug discovery and have established new evidence for regulatory drug approval, and the platform is being utilized to steadily deliver compatible therapeutic agents to patients. To date, 127 clinical trials (led by either companies or physicians) have been conducted to administer compatible therapeutic agents to subjects with gene alterations, and there have been over 1,200 registered cases of people who were enrolled in clinical trials based on genetic compatibility. Among the 32 trials that reached the stage of presenting final reports, 14 were granted regulatory approval (as of August 2022). As we can see, clinical trials conducted through SCRUM-Japan are obtaining regulatory approval rapidly and at a very high rate.
When the SCRUM-Japan initiative was launched in 2015, the initial focus was on the lungs and digestive tract, but that focus was expanded to include all solid tumors in 2019. These efforts have resulted in significantly less drug lag for solid tumors, but similar efforts in the field of hematologic cancers have yet to begin. Among therapeutic agents for hematologic cancers which were approved in Europe and the U.S. by the beginning of 2020, over 30 agents have not yet been approved in Japan.
Liquid biopsies are being utilized to improve precision cancer medicine
In 2019, SCRUM-Japan began using liquid biopsies as its main form of initial testing. While taking cancer tissue samples multiple times places a heavy burden on patients, liquid biopsies can be performed by taking blood samples to examine gene alterations in cancer cells. This means they lessen the burden on patients and provide results more rapidly. For example, to obtain test results for gastrointestinal cancer, cancer tissue tests take about one month while liquid biopsies take about ten days. In this manner, utilizing liquid biopsies contributes greatly to reducing turnaround time (TAT) and to improving clinical trial enrollment.
Liquid biopsies are also used to detect circulating tumor DNA, or ctDNA, which is cancer cell DNA that has leaked into the bloodstream due to causes like cell death. It has been found that there is almost no chance of recurrence in patients who have undergone surgery for solid tumors when no ctDNA is detected. This means that analyzing minimal residual disease (MRD) using postoperative liquid biopsies may make it possible for physicians to select treatments by recurrence risk. For example, they might decide to only administer adjuvant chemotherapy to reduce recurrence risk in patients who test positive for ctDNA. Clinical trials and other efforts aiming to optimize the use of adjuvant chemotherapy are currently advancing and expectations are high for the findings of such efforts to transform real-world clinical practice.
Focused efforts must be devoted to lowering the costs of drug discovery in precision cancer medicine
Challenges in the field of drug discovery for precision cancer medicine include (1) the small number of cases means extensive screening must be conducted to gather subjects, which is costly; and (2) drug discovery companies have less incentive to develop new drugs due to the small market size. Key methods of addressing these issues will include (a) making effective use of a “regulatory response registry,” which would involve establishing a mechanism for drugs to be approved by comparing them with reliable clinical data from outside parties, or Real World Data, without the need to implement comparative studies in clinical trials; and (b) relaxing the criteria for regulatory approval when expanding drug indications to include new cancer types. Efforts for rare diseases are also advancing through physician-led clinical trials.
The development of antibody-drug conjugates (ADCs) and middle molecule drugs are creating new possibilities for treatment
Although there have been recent advances in genomic cancer research, these alone have not led to the discovery of many new driver genes. However, there has also been a series of success stories in recent years in new classes of drugs like antibody-drug conjugates (ADCs), radiopharmaceuticals, and middle molecule drugs. In other words, there is an ongoing shift in the environment surrounding drug discovery in which emphasis is beginning to be placed on identifying therapeutic drug targets by analyzing a broad range of information including RNA and proteins (“multi-omics analysis”). This includes developing more effective antibody drugs by targeting cell membrane protein expression instead of gene alterations, or by developing molecularly targeted drugs for gene alterations that appear with high frequency and were not targeted for drug discovery in the past.
Within the area of new drug discovery technologies, two items have been attracting attention in recent years: (1) ADCs and (2) middle molecule drugs.
(1) ADCs combine anti-cancer agents with antibodies. One particularly well-known ADC is T-DXd (Trastuzumab Deruxtecan; brand name: ENHERTU), which is highly effective for a driver gene called HER2. T-DXd has been confirmed to have sufficient effect even in cases where HER2 levels are low, so it can be used to create treatment plans based only on HER2 immunostains. Doing so eliminates the need to conduct gene panel tests.
(2) Middle molecule drugs have molecular weights that are between those of general drugs with low molecular weights and conventional biopharmaceuticals. A representative example of a treatment using a middle molecule drug is peptide receptor radionuclide therapy, or PRRT, in which lutetium (177Lu) oxodotreotide (brand name: Lutathera) is utilized to effectively treat neuroendocrine tumors.
Expectations are also high for middle molecule drugs to be used to effectively treat KRAS gene alterations, which have been found in many cancers but have been untreatable.
In addition, 50 facilities throughout Japan are working together in an initiative called MONSTAR-SCREEN-2,[4] which aims to identify new drug discovery targets by collecting detailed data on changes in areas around tumors when drugs are administered and by conducting extensive tissue and blood analyses.
Legislation prohibiting discrimination based on genetic information should be enacted rapidly
Laws for the protection of genetic information must be formulated to prevent social discrimination based on genetic information. Such legislation has already been enacted in Europe and the United States. In Japan, one such bill was submitted by a nonpartisan Diet members’ group during the last extraordinary Diet session but was not discussed. I hope to see that bill enacted during the currently ordinary session of the Diet.
[1] “What are core hospitals for coordinated genomic cancer treatment?” (MHLW homepage): https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/gan/gan_byoin.html
[2] https://for-patients.c-cat.ncc.go.jp/
[3] http://www.scrum-japan.ncc.go.jp/
[4] http://www.scrum-japan.ncc.go.jp/monstar_screen/monsterscreen2/index.html
[Event Overview]
- Speaker: Atsushi Otsu (Hospital Director, National Cancer Center Hospital East)
- Date & time: Thursday, March 2; 18:30-20:00 JST
- Venue: Zoom webinar
- Language: Japanese
- Participation fee: Free
- Capacity: 500 participants
■ Profile:
Atsushi Otsu (Hospital Director, National Cancer Center Hospital East)
 Dr. Atsushi Otsu graduated from Tohoku University School of Medicine in 1983. After obtaining his doctorate in 1992, he has served as a gastrointestinal oncologist at the National Cancer Center Hospital East. In 1997, he left that role temporarily to study at MD Anderson Cancer Center in the U.S. In 2012, he was appointed Director of the Exploratory Oncology Research & Clinical Trial Center where he led efforts to develop systems for conducting research on new cancer therapeutics with First-in-Human (FIH) trials, investigator-initiated clinical trials, and translational research. From 2015 to 2017, he also served at the Japan Agency for Medical Research and Development (AMED) as a scientific and technical advisor. He assumed his current position of Hospital Director of the National Cancer Center Hospital East in 2016. To date, he has published over 300 English-language articles in publications like the New England Journal of Medicine, the Lancet, the Journal of Clinical Oncology, Lancet Oncology, the Journal of the National Cancer Institute. He is a member of the Japan Society of Clinical Oncology (JSCO) where he serves as Board Member and Chair of the International Committee; as well as the Japanese Cancer Association, where he serves as Board Member. He also serves as an expert member or evaluation committee member at the Pharmaceuticals and Medical Devices Agency (PMDA), the Ministry of Health, Labor and Welfare (MHLW), and the Ministry of Education, Culture, Sports, Science and Technology (MEXT).
Dr. Atsushi Otsu graduated from Tohoku University School of Medicine in 1983. After obtaining his doctorate in 1992, he has served as a gastrointestinal oncologist at the National Cancer Center Hospital East. In 1997, he left that role temporarily to study at MD Anderson Cancer Center in the U.S. In 2012, he was appointed Director of the Exploratory Oncology Research & Clinical Trial Center where he led efforts to develop systems for conducting research on new cancer therapeutics with First-in-Human (FIH) trials, investigator-initiated clinical trials, and translational research. From 2015 to 2017, he also served at the Japan Agency for Medical Research and Development (AMED) as a scientific and technical advisor. He assumed his current position of Hospital Director of the National Cancer Center Hospital East in 2016. To date, he has published over 300 English-language articles in publications like the New England Journal of Medicine, the Lancet, the Journal of Clinical Oncology, Lancet Oncology, the Journal of the National Cancer Institute. He is a member of the Japan Society of Clinical Oncology (JSCO) where he serves as Board Member and Chair of the International Committee; as well as the Japanese Cancer Association, where he serves as Board Member. He also serves as an expert member or evaluation committee member at the Pharmaceuticals and Medical Devices Agency (PMDA), the Ministry of Health, Labor and Welfare (MHLW), and the Ministry of Education, Culture, Sports, Science and Technology (MEXT).
Top Research & Recommendations Posts
- [Policy Recommendations] The Path to a Sustainable Healthcare System: Three Key Objectives for Public Deliberation (January 22, 2026)
- [Research Report] The 2025 Public Opinion Survey on Healthcare in Japan (March 17, 2025)
- [Research Report] Perceptions, Knowledge, Actions and Perspectives of Healthcare Organizations in Japan in Relation to Climate Change and Health: A Cross-Sectional Study (November 13, 2025)
- [Policy Recommendations] Reshaping Japan’s Immunization Policy for Life Course Coverage and Vaccine Equity: Challenges and Prospects for an Era of Prevention and Health Promotion (April 25, 2025)
- [Research Report] The 2023 Public Opinion Survey on Satisfaction in Healthcare in Japan and Healthcare Applications of Generative AI (January 11, 2024)
- [Public Comment Submission] “Assessment Report on Climate Change Impacts in Japan (Draft Overview)” (December 24, 2025)
- [Policy Recommendations] Developing a National Health and Climate Strategy for Japan (June 26, 2024)
- [Research Report] The Public Opinion Survey on Child-Rearing in Modern Japan (Final Report) (March 4, 2022)
- [Research Report] Survey of Japanese Physicians Regarding Climate Change and Health (December 3, 2023)
- [Policy Recommendations] Achieving Equity in Multidisciplinary Pain Treatment and Support Systems for Pain Management (March 31, 2023)
Featured Posts
-
2026-01-09
[Registration Open] (Hybrid Format) Dementia Project FY2025 Initiative Concluding Symposium “The Future of Dementia Policy Surrounding Families and Others Who Care for People with Dementia” (March 9, 2026)
![[Registration Open] (Hybrid Format) Dementia Project FY2025 Initiative Concluding Symposium “The Future of Dementia Policy Surrounding Families and Others Who Care for People with Dementia” (March 9, 2026)](https://hgpi.org/en/wp-content/uploads/sites/2/dementia-20260309-top.png)
-
2026-02-05
[Registration Open] (Webinar) The 141st HGPI Seminar “Current Status and Future Prospects of Korea’s Obesity Policy: Voices of People with Lived Experience in Policy Promotion” (March 3, 2026)
![[Registration Open] (Webinar) The 141st HGPI Seminar “Current Status and Future Prospects of Korea’s Obesity Policy: Voices of People with Lived Experience in Policy Promotion” (March 3, 2026)](https://hgpi.org/en/wp-content/uploads/sites/2/hs141-top-1.png)
-
2026-02-06
[Research Report] AMR Policy Update #5: Cancer Care and AMR (Part 2)
![[Research Report] AMR Policy Update #5: Cancer Care and AMR (Part 2)](https://hgpi.org/en/wp-content/uploads/sites/2/HGPI_20260204_AMR-Policy-Update-5.png)