[Event Report] The 58th Special Breakfast Meeting “Challenges and Prospects for the 3rd Phase of AMED” (July 15, 2025)
date : 9/25/2025
![[Event Report] The 58th Special Breakfast Meeting “Challenges and Prospects for the 3rd Phase of AMED” (July 15, 2025)](https://hgpi.org/en/wp-content/uploads/sites/2/0L6A4976_web-1.jpg)
The 58th Special Breakfast Meeting featured President of the Japan Agency for Medical Research and Development (AMED), Professor Hitoshi Nakagama.
Professor Nakagama, who assumed his position as President of AMED in April 2025, discussed AMED’s past achievements and the direction for its initiatives during its 3rd Phase.
<Key points of the lecture>
- In the ten years since AMED was founded, it has built a system that provides unified support for medical research from basic research to practical application. Annually, AMED invests approx. 160 billion yen to advance a wide range of studies from basic research to practical application, with its focus mainly on supporting research that is near the practical application phase. By establishing the Strategic Center of Biomedical Advanced Vaccine Research and Development for Preparedness and Response (SCARDA) and strengthening global collaboration, AMED is also advancing efforts to create a system for infectious disease preparedness with a view toward global expansion. Support provided through this system is focused on the practical application of research findings.
• During the 3rd Phase of AMED, which began in April 2025, AMED will remain centered upon and expand the six Integrated Projects from the 2nd Phase, and will promote the practical application of findings through the effective use of data and clinical research acceleration with a focus on areas including drug discovery, medical devices, regenerative medicine, and infectious disease. With regards to operations, AMED will expand collaboration among projects, opportunities for co-creation with industry and academia, and basic research to reinforce systems that lead to the efficient application of noteworthy basic research assets centered on international expansion and healthcare digital transformation (DX).
• Moving forward, AMED aims to serve as a link between the domestic drug discovery ecosystem and the international drug discovery ecosystem by collaborating with venture capital (VC) firms from Japan and overseas and by promoting international brain circulation. Moving forward, AMED will establish frameworks that bridge public and private funding to strengthen Japan’s drug discovery ecosystem in Japan and overseas.
■Overview of AMED and achievements from its 1st and 2nd Phases
By providing the health sector with unified support for R&D from basic research to practical application, AMED’s activities aim to assist progress in R&D and to establish an environment that can be used effectively in health sector R&D. It has been ten years since AMED was established, and it is currently in its 3rd Phase, which began in April 2025.
Results in R&D promotion
Annually, AMED invests approx. 160 billion yen to support approx. 2,600 research proposals, about 25% to 30% of which are adopted as new projects. As for the distribution of research funds, the largest number of proposals are provided with 10 million yen to 25 million yen annually, followed by those provided with 25 million yen to 50 million yen annually. This occurs because vast funding becomes required as research aiming for practical application advances into the later stages of development. One characteristic of AMED is that its research grants are more generous than other public organizations. In fact, obtaining research funding from AMED requires applicants to spend an average of about two years and eleven months to prepare and accumulate findings through research conducted through other sources of funding. This has created circumstances in which research projects apply for and are adopted as AMED projects after accumulating findings and reaching the stage where their focus is turned to practical application.
At the same time, AMED also recognizes the importance of frameworks that produce results while fostering research, and the ratio of proposals that are adopted is higher for those in the development phase compared to the basic research and practical application phases. Around 60% of AMED’s research funds are provided to universities and other research institutions, followed by independent administrative agencies, national testing and research institutions, and private sector companies. By area of disease, AMED’s main focus is cancer followed by infectious disease control, including for emerging and reemerging infectious diseases. Other areas where AMED devotes a significant amount of research funding include cardiovascular disease and neurological disease.
Examining major achievements of AMED projects in detail, over 6,000 papers in basic research have been published, 357 proofs of concept (PoC) in applied research have been acquired, and 434 clinical trials have been conducted. Among those trials, 40 received regulatory approval and have achieved practical application. Expectations are high for AMED to clearly indicate where the “exits” are for research and to support research in a cross-cutting manner that spans all modalities or drug discovery methods.
The establishment of the Strategic Center for Advanced Research and Development (SCARDA)
In recognition of the importance of infectious disease crisis preparedness, the Strategic Center of Biomedical Advanced Vaccine Research and Development for Preparedness and Response (SCARDA) was established within AMED in accordance with the Strategy for Strengthening the Vaccine Development and Production System approved by Cabinet Decision on June 1, 2021.
The three key functions of SCARDA are: (1) gathering and analyzing a broad scope of information; (2) performing strategic decision-making; and (3) providing flexible funding. To promote vaccine development as part of the vaccine strategy, 51.5 billion yen has been allocated to the “Japan Initiative for World-leading Vaccine Research and Development Centers,” 150 billion yen to the “Program on R&D of new generation vaccines including new modality application,” and 50 billion yen to the “Strengthening Program for Pharmaceutical Startup Ecosystem.” In this manner, SCARDA is working to ensure Japan has a system in place for the rapid development of therapeutics in the event of an emergency.
Initiatives from Japan in global collaborative research
Currently, there are concerns about the decline of Japan’s global standing in science and technology. In addition to providing financial support, expectations are high for AMED to help address those concerns by also providing support that expands international brain circulation, international collaboration, and international joint research for the practical application of research findings.
For example, Japan has been collaborating with the U.S. National Institutes of Health (NIH) for over 60 years, starting with the establishment of the U.S.-Japan Cooperative Medical Sciences Program (USJCMSP) in 1965. As for collaboration with Europe, AMED has signed a cooperative agreement on infectious diseases with the Health Emergency Preparedness and Response Authority of the European Commission (HERA). AMED has also built cooperative frameworks with various countries for joint research based on Memorandums of Cooperation (MOC) or the e-ASIA Joint Research Program.
■The direction of AMED efforts in its 3rd Phase
AMED’s approach to further promoting R&D through Integrated Projects
While reflecting on past achievements and challenges, the Integrated Project framework centered on items like modalities adopted during the 2nd Phase will generally remain in place during the 3rd Phase. This will allow for consistency in research to be maintained while enabling efforts for further progress and practical application. Specifically, AMED has advanced R&D in various areas of disease under six Integrated Projects: “Innovative Drug Discovery and Development,” “Medical Devices and Healthcare,” “Regenerative Medicine and Cell and Gene Therapies,” “Genome and Health-Related Data,” “Basic Medical Research,” and “Basic Research for Medical Innovation.” In addition to contributing to further clarifying development objectives and to fostering exit-oriented attitudes toward research among researchers, the Integrated Project framework has also reinforced collaboration among projects.
During its 3rd Phase, AMED will retain parts of this integrated project framework, with “Innovative Drug Discovery and Development,” “Medical Devices and Healthcare,” “Regenerative Medicine and Cell and Gene Therapies,” and “Infectious Disease Research” serving as major projects (see Fig. 1). We will also expand the Project for Data Utilization and Life Course Research and Development, which supports these major projects. This will aim to bridge gaps to practical application and accelerate clinical development through collaboration with basic research asset development and basic research. Finally, there are high expectations for AMED to spur innovation and reinforce the drug discovery ecosystem to serve as “exits” for R&D. Disease areas will be broadly arranged into three categories: cancer, intractable and rare diseases, and the life course (which encompasses lifestyle diseases and other diseases related to risks that accumulate at each life stage).
AMED’s total budget for FY2025 is expected to be approx. 200 billion yen. This includes an initial budget of 123.2 billion yen and an adjustment budget of 17.5 billion yen to be allocated from endowment projects and the President’s discretionary funds. As for how the budget will be allocated, the largest portion will be allocated to the “Innovative Drug Discovery and Development” project.
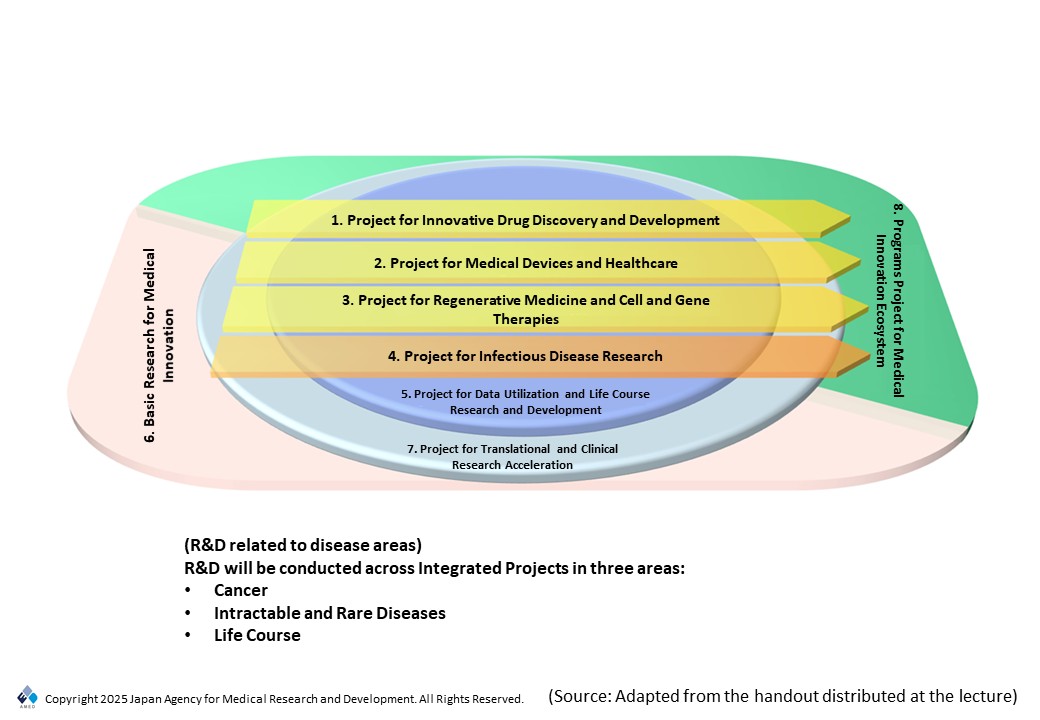
The future direction of AMED operations
AMED has set the following directions to guide its operations during its 3rd Phase.
- Strengthen collaboration among projects
Instead of fostering each project individually, AMED aims to enable a smooth transition to practical application by addressing issues present between each phase of R&D by strengthening collaboration among projects. To do this, AMED is currently advancing efforts to build and introduce internal mechanisms for pairing and matching. - Collaborate with industry and academia and engage in private licensing from the initial stages of R&D
AMED will work to increase the probability that projects successfully reach practical application by involving companies from the initial stages of R&D. - Enhance basic research to generate results that lead to widespread adoption in and contribute to society
Given the importance of advancing basic research while keeping widespread adoption in society in mind, AMED aims to provide continuous and stable support to enhance basic research that contributes to generating results. - Promote international expansion
Making full use of its global research network to deepen collaboration in basic research asset development and among projects, AMED will establish a foundation for producing excellent research results. It will also be important for AMED to continuously and actively contribute to building an environment in which the domestic pharmaceutical market attracts international attention by advancing domestic R&D that proactively incorporates global needs. - Advance the digital transformation of R&D in the health sector
Utilizing technologies such as artificial intelligence (AI) and quantum technology, AMED aims to contribute to creating a society of healthy longevity by producing and verifying new evidence based on big data and the effective use of personal health records.
Based on these principles, AMED aims to build a system for promoting the practical application of promising basic research assets with greater efficiency by gathering information, including knowledge from other countries; by addressing issues related to collaboration across projects; and by providing support that serves as a bridge for companies.
■AMED’s recent initiatives
Utilizing discretionary funds to reinforce existing research projects and accelerate the establishment of new projects
The AMED President’s discretionary funds are an adjustment budget used to reinforce existing research projects and to accelerate the establishment of new projects. Effective methods of using these funds are determined through internal discussions at AMED as well as through consultations with relevant ministries and agencies. The three items described below provide specific examples of how these funds are being utilized in FY2025.
- Developing a system program to support surgical planning for pediatric patients with congenital heart disease
Significant variation in cardiovascular structure means pediatric patients require personalized surgical planning. With sights set on the international expansion of the program that this initiative is developing, discretionary funds are being used to support the development of clinical trial protocols that comply with other countries’ pharmaceutical regulations. We hope that this will encourage other countries to adopt Japan’s innovative medical support systems. - R&D related to elucidating the pathophysiology of schizophrenia
Aiming to elucidate the pathophysiology of schizophrenia, studies on synaptic behavior during memory formation and on molecules related to that process are now advancing. The discretionary funds are being utilized to promote collaboration with another research group that has developed a new method of molecular design in hopes that it will advance efforts to identify or develop new therapeutic targets and therapeutics for schizophrenia. - Establishing and expanding a foundation for rapid genetic diagnosis in critically ill newborns
Japan’s diagnostic infrastructure only had one center for rapid genetic diagnosis in critically ill newborns, which was located in eastern Japan. There were also issues related to systems for data integrity and response. Discretionary funds were used to establish a new center in western Japan and plans are in place to reinforce data security and to strengthen the system for genetic counseling. Expectations are high for these efforts to accelerate and streamline genome diagnosis and elevate diagnosis rates.
Strengthening the drug discovery ecosystem
Achieving the practical application of research findings or bringing research results to market require vast amounts of funding. However, as it is difficult for AMED to fully cover these costs with public funds, cooperation from venture capital (VC), investors, and pharmaceutical companies is required. To support efforts to raise private funding in the early stages of research, the domestic drug discovery ecosystem must be strengthened. Given these circumstances, AMED is currently advancing the new initiatives described below.
Promoting human resource development and international brain circulation
AMED recognizes a gap in coordination that links researchers with VC, investors, and pharmaceutical companies, as well as an insufficient human resource pool for filling coordinator roles. By proactively recruiting international human resources, AMED is advancing efforts to expand the domestic drug discovery ecosystem.
(Photo: Kazunori Izawa)
Collaborating with registered VCs
Based on their track records of investment in drug discovery, AMED has selected 30 VCs as “registered VCs.” By combining support from registered VCs with the subsidies AMED provides to drug discovery startups, AMED is working to accelerate drug development. As around half of the registered VCs are international firms, this initiative is helping to create opportunities to inform the international community about basic research assets from Japan and to acquire support from overseas investors.
Linking national and international ecosystems
While serving as a foundation for fostering basic research assets in Japan, it is also important for AMED to promote global drug discovery through collaboration with drug discovery ecosystems overseas. AMED is expanding activities in which it steadily provides thorough introductions of Japan’s basic research assets to related institutions, investors, and other such parties. These efforts are expected to contribute to the development of Japan’s drug discovery ecosystem as well as to encouraging further participation from domestic Japanese pharmaceutical companies.
The lecture was followed by a Q&A session with the audience. Participants held a lively discussion on topics including the need to strengthen the evaluation system to support practical application, specific details on reinforcing systems to promote the international expansion of initiatives, methods of developing human resources, and fields of medical research in which the utilization of DX should be advanced.
(Photography: Kazunori Izawa)
■Profile
Hitoshi Nakagama (President, Japan Agency for Medical Research and Development (AMED))
Professor Hitoshi Nakagama graduated from the University of Tokyo Faculty of Medicine in 1982. In 1990, he was appointed Assistant Professor at the Third Department of Internal Medicine at that same university. The following year, in 1991, he began serving as a Research Fellow at the Massachusetts Institute of Technology (MIT) Center for Cancer Research (CCR) in the US. He was awarded a Doctor of Medicine degree in 1992. At the National Cancer Center Research Institute (NCCRI), he was appointed Director of the Division of Carcinogenesis in 1995, and later went on to serve as Director, Department of Biochemistry; Deputy Director; and Director. He was appointed Chief Director and President of the National Cancer Center in April 2016 and President of the Japan Agency for Medical Research and Development (AMED) in April 2025. His research is centered on the analysis of environmental and genetic factors of human carcinogenesis and the molecular mechanisms of those factors. His areas of specialty are molecular oncology, cancer genomics, and environmental carcinogenesis.
Top Research & Recommendations Posts
- [Policy Recommendations] The Path to a Sustainable Healthcare System: Three Key Objectives for Public Deliberation (January 22, 2026)
- [Research Report] The 2025 Public Opinion Survey on Healthcare in Japan (March 17, 2025)
- [Research Report] Perceptions, Knowledge, Actions and Perspectives of Healthcare Organizations in Japan in Relation to Climate Change and Health: A Cross-Sectional Study (November 13, 2025)
- [Policy Recommendations] Reshaping Japan’s Immunization Policy for Life Course Coverage and Vaccine Equity: Challenges and Prospects for an Era of Prevention and Health Promotion (April 25, 2025)
- [Research Report] The 2023 Public Opinion Survey on Satisfaction in Healthcare in Japan and Healthcare Applications of Generative AI (January 11, 2024)
- [Public Comment Submission] “Assessment Report on Climate Change Impacts in Japan (Draft Overview)” (December 24, 2025)
- [Policy Recommendations] Developing a National Health and Climate Strategy for Japan (June 26, 2024)
- [Research Report] The Public Opinion Survey on Child-Rearing in Modern Japan (Final Report) (March 4, 2022)
- [Research Report] Survey of Japanese Physicians Regarding Climate Change and Health (December 3, 2023)
- [Policy Recommendations] Achieving Equity in Multidisciplinary Pain Treatment and Support Systems for Pain Management (March 31, 2023)
Featured Posts
-
2026-01-09
[Registration Open] (Hybrid Format) Dementia Project FY2025 Initiative Concluding Symposium “The Future of Dementia Policy Surrounding Families and Others Who Care for People with Dementia” (March 9, 2026)
![[Registration Open] (Hybrid Format) Dementia Project FY2025 Initiative Concluding Symposium “The Future of Dementia Policy Surrounding Families and Others Who Care for People with Dementia” (March 9, 2026)](https://hgpi.org/en/wp-content/uploads/sites/2/dementia-20260309-top.png)
-
2026-02-05
[Registration Open] (Webinar) The 141st HGPI Seminar “Current Status and Future Prospects of Korea’s Obesity Policy: Voices of People with Lived Experience in Policy Promotion” (March 3, 2026)
![[Registration Open] (Webinar) The 141st HGPI Seminar “Current Status and Future Prospects of Korea’s Obesity Policy: Voices of People with Lived Experience in Policy Promotion” (March 3, 2026)](https://hgpi.org/en/wp-content/uploads/sites/2/hs141-top-1.png)
-
2026-02-06
[Research Report] AMR Policy Update #5: Cancer Care and AMR (Part 2)
![[Research Report] AMR Policy Update #5: Cancer Care and AMR (Part 2)](https://hgpi.org/en/wp-content/uploads/sites/2/HGPI_20260204_AMR-Policy-Update-5.png)




